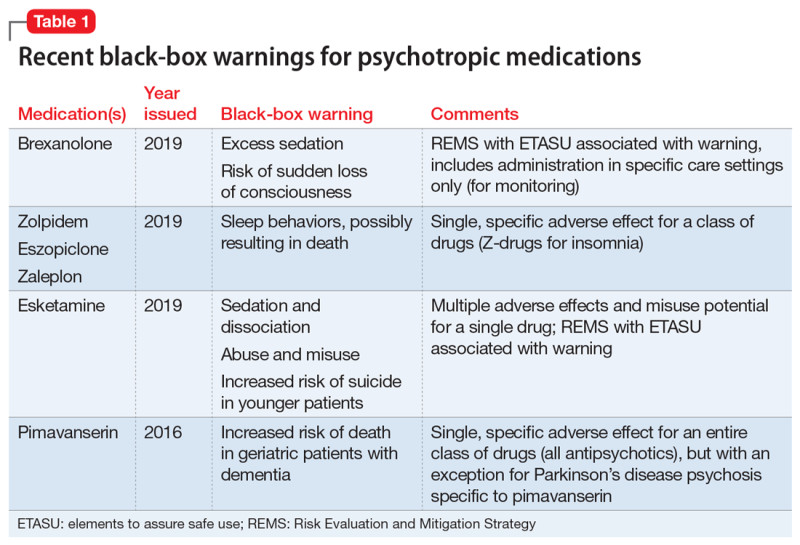
In a move that's stirring conversation among women's health advocates, the U.S. Food and Drug Administration (FDA) has announced plans to eliminate longstanding black box warnings from hormone replacement therapy (HRT) products used to treat menopause symptoms. These stark alerts, plastered on creams, pills, and patches for over two decades, highlighted risks like heart disease, blood clots, breast cancer, and even dementia—findings rooted in the 2002 Women's Health Initiative study that involved thousands of women averaging 63 years old at the time.
The decision comes as experts argue the warnings overstated dangers, especially for younger women starting HRT near menopause onset. Recent analyses show that when initiated within a decade of menopause, HRT can cut hot flash severity by up to 80% and bolster bone health without the same elevated risks. FDA Commissioner Marty Makary emphasized this reevaluation during a recent briefing, noting the agency's push to update labels based on evolving science rather than outdated data.
Still, not all warnings are vanishing; estrogen-only therapies retain their endometrial cancer alert. This change, coordinated with the Department of Health and Human Services, aims to empower more informed choices for the roughly 1 million U.S. women entering menopause annually. Critics, though, urge caution, pointing to individual variability in risk profiles.
As guidelines evolve, how might this reshape conversations around menopause care in your own life?